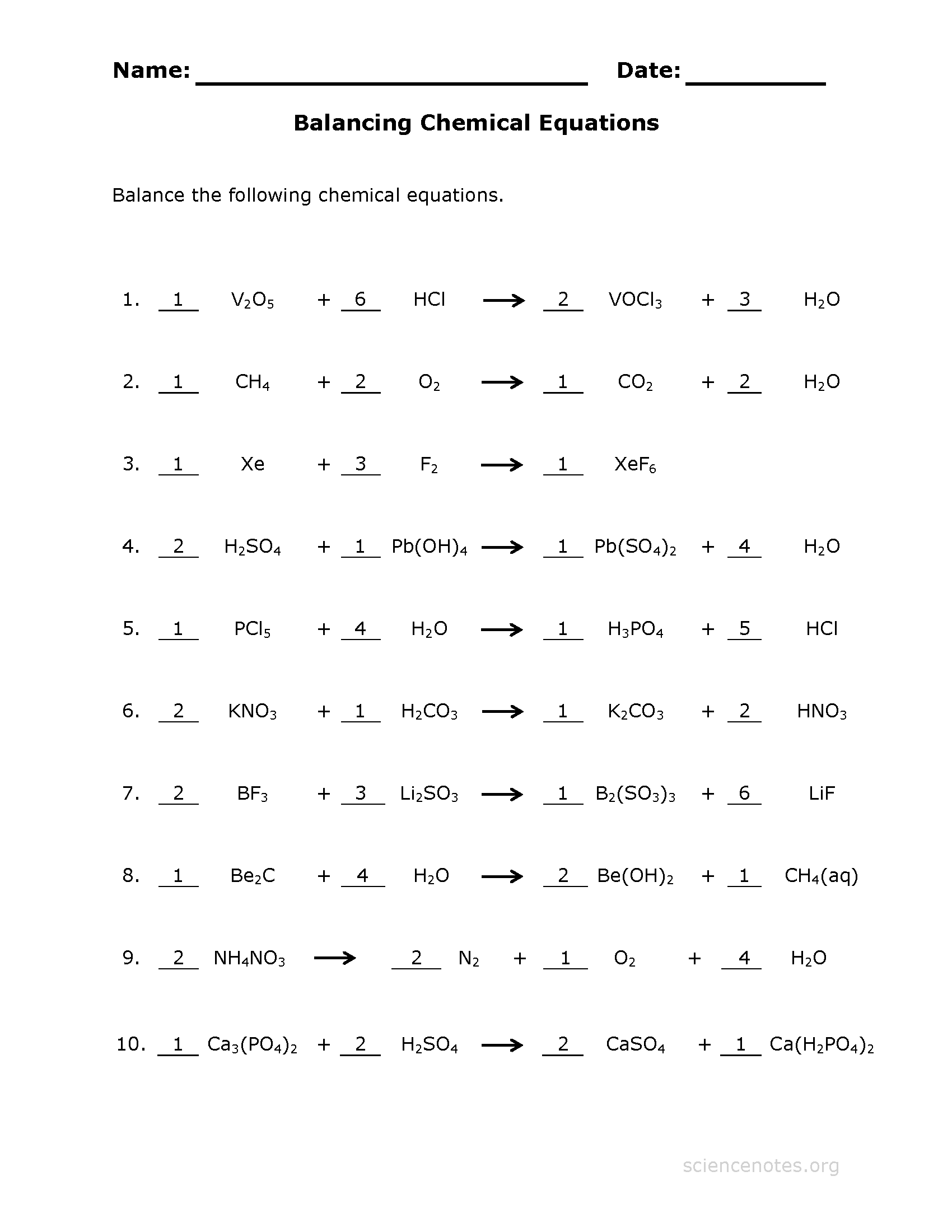
Balancing Chemical Equations Worksheet 3 Answer Key - Web balance the equations below: _1_ h3po4 + _3__ koh æ _1__ k3po4 + _3__ h2o. _6__ k + _1__ b2o3 æ _3__ k2o. Once you’ve done it a few times, it becomes easier and easier. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3. 1) ____ n2 + ____ h2 ____ nh3 2) ____ kclo3 ____ kcl + ____ o2 3) ____ nacl + ____ f2 ____ naf + ____ cl2 4) ____ h2 + ____ o2 ____. Web balancing chemical equations requires practice. 2 fe+ 3 cl2 − −→ 2 fecl3. (coefficients equal to one (1) do not need to be shown in your answers).
49 Balancing Chemical Equations Worksheets [with Answers]
(coefficients equal to one (1) do not need to be shown in your answers). 1) ____ n2 + ____ h2 ____ nh3 2) ____ kclo3 ____ kcl + ____ o2 3) ____ nacl + ____ f2 ____ naf + ____ cl2 4) ____ h2 + ____ o2 ____. Web balancing chemical equations requires practice. Web balance the equations below: Once.
49 Balancing Chemical Equations Worksheets With Answers —
(coefficients equal to one (1) do not need to be shown in your answers). 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3. Web balancing chemical equations requires practice. _1_ h3po4 + _3__ koh æ _1__ k3po4 + _3__ h2o. 2 fe+ 3 cl2 − −→ 2 fecl3.
Worksheet More Practice Balancing Equations Balance The Following
(coefficients equal to one (1) do not need to be shown in your answers). Web balance the equations below: _1_ h3po4 + _3__ koh æ _1__ k3po4 + _3__ h2o. Web balancing chemical equations requires practice. Once you’ve done it a few times, it becomes easier and easier.
49 Balancing Equation Worksheet With Answers
(coefficients equal to one (1) do not need to be shown in your answers). Web balancing chemical equations requires practice. Web balance the equations below: Once you’ve done it a few times, it becomes easier and easier. 2 fe+ 3 cl2 − −→ 2 fecl3.
Chemistry Balancing Chemical Equations Worksheet Answer Key
2 fe+ 3 cl2 − −→ 2 fecl3. Web balance the equations below: Web balancing chemical equations requires practice. _6__ k + _1__ b2o3 æ _3__ k2o. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3.
20 Balancing Chemical Equations Worksheets (+ Answers)
1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3. Once you’ve done it a few times, it becomes easier and easier. Web balancing chemical equations requires practice. Web balance the equations below: 2 fe+ 3 cl2 − −→ 2 fecl3.
Balancing Chemical Equations Worksheet Answers
Web balancing chemical equations requires practice. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3. (coefficients equal to one (1) do not need to be shown in your answers). Web balance the equations below: 1) ____ n2 + ____ h2 ____ nh3 2) ____ kclo3 ____ kcl + ____ o2.
Balancing Chemical Equations Worksheet 3 Answer Key
2 fe+ 3 cl2 − −→ 2 fecl3. Web balance the equations below: _6__ k + _1__ b2o3 æ _3__ k2o. _1_ h3po4 + _3__ koh æ _1__ k3po4 + _3__ h2o. 1) ____ n2 + ____ h2 ____ nh3 2) ____ kclo3 ____ kcl + ____ o2 3) ____ nacl + ____ f2 ____ naf + ____ cl2 4).
49 Balancing Chemical Equations Worksheets [with Answers]
(coefficients equal to one (1) do not need to be shown in your answers). Web balance the equations below: 2 fe+ 3 cl2 − −→ 2 fecl3. Once you’ve done it a few times, it becomes easier and easier. _6__ k + _1__ b2o3 æ _3__ k2o.
Balance Chemical Equations Worksheet 3 Answer Key Science Notes and
2 fe+ 3 cl2 − −→ 2 fecl3. _6__ k + _1__ b2o3 æ _3__ k2o. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3. Web balance the equations below: _1_ h3po4 + _3__ koh æ _1__ k3po4 + _3__ h2o.
Web balancing chemical equations requires practice. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3. (coefficients equal to one (1) do not need to be shown in your answers). Once you’ve done it a few times, it becomes easier and easier. _6__ k + _1__ b2o3 æ _3__ k2o. 1) ____ n2 + ____ h2 ____ nh3 2) ____ kclo3 ____ kcl + ____ o2 3) ____ nacl + ____ f2 ____ naf + ____ cl2 4) ____ h2 + ____ o2 ____. Web balance the equations below: _1_ h3po4 + _3__ koh æ _1__ k3po4 + _3__ h2o. 2 fe+ 3 cl2 − −→ 2 fecl3.
_6__ K + _1__ B2O3 Æ _3__ K2O.
_1_ h3po4 + _3__ koh æ _1__ k3po4 + _3__ h2o. 1) ____ n2 + ____ h2 ____ nh3 2) ____ kclo3 ____ kcl + ____ o2 3) ____ nacl + ____ f2 ____ naf + ____ cl2 4) ____ h2 + ____ o2 ____. Once you’ve done it a few times, it becomes easier and easier. Web balance the equations below:
1) 1 N 2 + 3 H 2 2 Nh 3 2) 2 Kclo 3 2 Kcl + 3.
Web balancing chemical equations requires practice. (coefficients equal to one (1) do not need to be shown in your answers). 2 fe+ 3 cl2 − −→ 2 fecl3.

![49 Balancing Chemical Equations Worksheets [with Answers]](https://i2.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-11.jpg)






![49 Balancing Chemical Equations Worksheets [with Answers]](https://i2.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-25.jpg?w=395)